Introduction: Standard first line therapy (LOT) for patients (pts) with large B-cell lymphoma (LBCL) involves chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) or a similar regimen, e.g. R-CHOP with the addition of etoposide (R-EPOCH). While most pts respond, 30-40% of pts relapse or fail to achieve remission with 1st LOT. For those pts, the goal of curative 2nd LOT is typically platinum-based salvage chemotherapy in preparation for autologous stem cell transplant (ASCT). However, many pts (50% or more) do not qualify for intensive therapy, either due to age or comorbidities, or because they do not respond to or cannot tolerate salvage chemotherapy. For those further treatment options are limited.
Objectives: In a sample of U.S. LBCL pts 65 and older, who have progressed to 2nd LOT and are eligible for ASCT, to compare the patient characteristics, treatments, costs and overall survival between pts who received SCT with those who did not.
Methods: This retrospective cohort study used 100% Medicare Fee-for-Service Parts A/B claims data and Part D Prescription Drug Event data to identify pts ≥ 65 years of age, newly diagnosed with LBCL in 2012-2017, and who had ≥ 6 months continuous enrollment pre-diagnosis (baseline) and ≥ 12 months post-diagnosis, or who died after initiation of 2nd LOT. All were treated with a CHOP-like regimen 1st LOT and progressed to 2nd LOT.
Eligible pts who received a 2nd LOT with a SCT-preparative regimen per NCCN guidelines were stratified into the "ASCT-intended" cohort, and the remainder into the "ASCT-not-intended" cohort. The ASCT-intended group was further subset into those who received a SCT and those who did not (Non-SCT). These 2 subsets formed the primary comparison groups.
Baseline characteristics were age, gender, race, dual eligibility status (i.e. Medicare plus Medicaid), and Charlson-Deyo Comorbidity Index (CCI). Measures of utilization during follow-up (from initiation of 2nd LOT to loss to follow-up) were hospitalizations, outpatient and emergency department (ED) visits, and post-acute care. Costs during follow-up reflected standardized, inflation-adjusted, all-cause paid amounts. Utilization and cost metrics were standardized to per-patient-per-month (PPPM). Overall survival (OS) was measured from the start of 2nd LOT until death, disenrollment or the end of the study period.
Results: 4,758 pts met all inclusion criteria. 3,713 (78%) did not receive a SCT-preparative regimen after 1st LOT. 1,045 pts (22%) received a SCT-preparative regimen and constituted the ASCT-intended group. Of these, 244 (23.3%) received a SCT and 801 (76.7%) did not. The remainder of this report focuses on a comparison of the SCT and non-SCT cohorts.
Baseline characteristics, treatments, utilization and cost are summarized in Table 1. Both groups were predominantly white, but SCT pts were, on average, 5 years younger, slightly more likely to be male, and had slightly lower mean CCI scores. 13.1% of non-SCT pts were dual eligible vs. 4.5% of SCT (all p < .01 except Race).
93.9% of SCT procedures were autologous, and no pts had more than 1 SCT during follow-up.
Non-SCT pts had higher all-cause utilization for all categories except for acute hospitalizations. The higher inpatient utilization for the SCT group may reflect the SCT procedure itself (94% of SCT were inpatient). A similar pattern was observed in the cost data, where Non-SCT pts incurred higher costs except for acute hospitalizations.
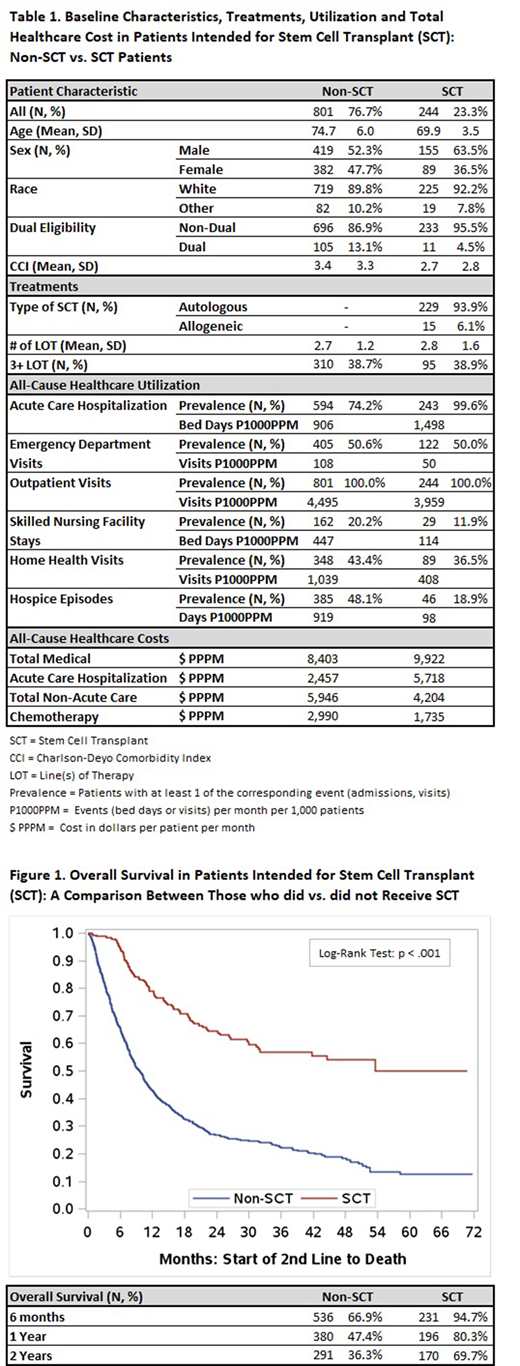
Results for OS are summarized in Figure 1. Non-SCT pts had significantly lower rates of survival than SCT pts. 94.7% of SCT pts were still alive 6 months after initiation of 2nd LOT, whereas only 66.9% of non-SCT pts were. At two years post-2nd LOT, survival rates were 69.7% vs. 36.3%.
Conclusions: In this study, only 23.3% of SCT-intended pts ultimately received it. The remaining 76.7% were SCT-intended but never received a transplant. Compared to the SCT pts, the non-SCT pts were older, had more comorbidities, had higher rates of healthcare utilization and costs post-2nd line therapy in all categories (except acute inpatient), and lower OS. 38.7% of non-SCT pts proceeded to additional lines of lymphoma-directed therapies beyond 2nd line, which may suggest that their clinicians felt that there was potential benefit to further treatment. These data suggest there may be unmet need for elderly pts with LBCL who are eligible for ASCT, but do not ultimately receive it.
Kilgore:Kite, A Gilead Company: Research Funding. Wong:Kite, A Gilead Company: Research Funding. Thornton Snider:Precision Medicine Group: Ended employment in the past 24 months, Research Funding; Gilead Sciences: Current equity holder in publicly-traded company, Research Funding; Kite, A Gilead Company: Current Employment. Cheng:Kite, A Gilead Company: Current Employment; Gilead Sciences: Current equity holder in publicly-traded company. Schroeder:Kite, A Gilead Company: Research Funding. Mohammadi:Kite, A Gilead Company: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract